
The Periodic Table: Exploring Elements, Patterns, and Applications
Discover the beauty and significance of the periodic table, its organized structure, and the vibrant color scheme that aids in understanding the elements. Learn about its applications in various scientific fields and its ongoing role in research and discoveries.
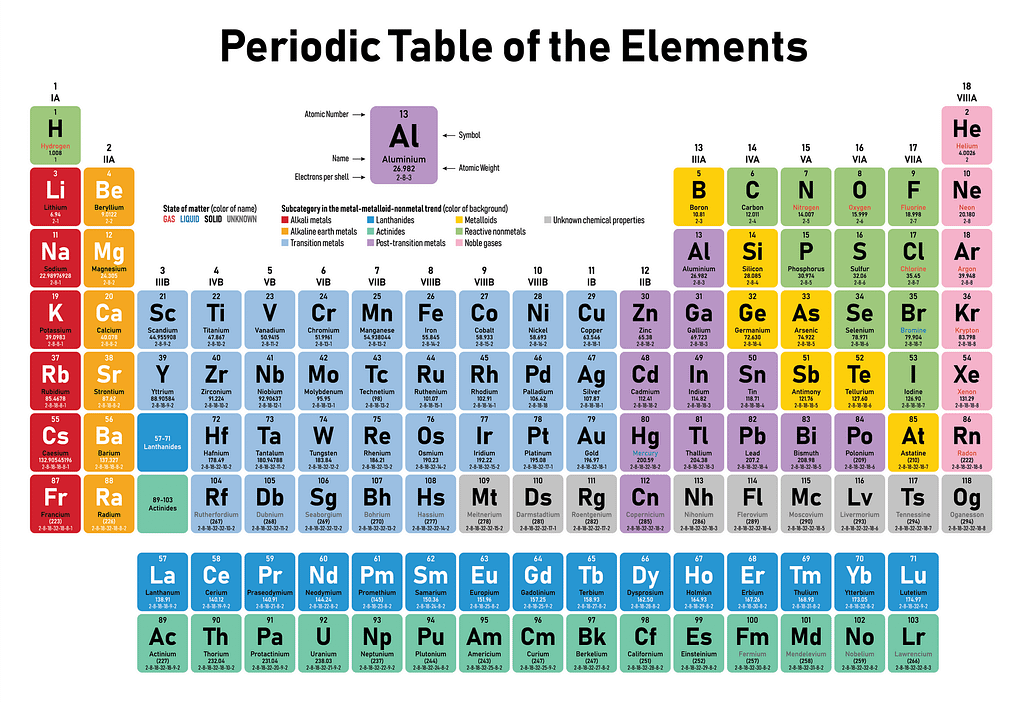
The Periodic Table: A Colorful Guide to the Elements
Introduction
The periodic table is a cornerstone of chemistry, serving as a comprehensive guide to the elements that make up our universe. It is a masterpiece of organization, categorizing elements based on their properties and providing scientists and students with a wealth of information. With its vivid and colorful layout, the periodic table not only presents essential scientific knowledge but also captivates our imagination.
Structure and Organization
The periodic table consists of rows, called periods, and columns, known as groups or families. Each element is represented by a unique symbol and arranged in order of increasing atomic number. The atomic number corresponds to the number of protons in an atom’s nucleus, which defines an element’s identity.
The groups are numbered from 1 to 18, and each group shares similar chemical properties. For instance, the noble gases in Group 18 are known for their low reactivity, while alkali metals in Group 1 are highly reactive. The periods, on the other hand, represent the electron shells or energy levels of the atoms, with each period corresponding to a new shell being filled with electrons.
Colorful Representation
One of the most striking features of the periodic table is its vibrant and eye-catching color scheme. Different colors are used to highlight various categories and properties of the elements, making it easier to understand and interpret the information presented.
| Color | Element Category |
|---|---|
| Red | Nonmetals |
| Green | Alkali Metals |
| Purple | Alkaline Earth Metals |
| Yellow | Transition Metals |
| Blue | Halogens |
| Orange | Lanthanides and Actinides |
These colors not only add visual appeal to the table but also aid in identifying patterns and trends among the elements. They provide a quick visual reference, making it easier to comprehend the vast amount of information contained within the periodic table.
Importance and Applications
The periodic table serves as a fundamental tool for chemists, physicists, and other scientists. It allows them to predict and understand the behavior of elements, their compounds, and their reactions. The table enables scientists to explore relationships between elements, identify trends, and discover new compounds and materials.
Furthermore, the periodic table plays a vital role in various scientific and practical applications:
Chemical Research and Analysis:
Scientists rely on the periodic table to conduct research and analyze chemical reactions. It provides valuable information about an element’s properties, including its atomic radius, electronegativity, and valence electrons. This knowledge helps in designing experiments, predicting reaction outcomes, and developing new chemical compounds.
Material Science and Engineering:
Understanding the periodic table allows material scientists and engineers to explore and manipulate the properties of different elements and create new materials with specific characteristics. For example, the use of transition metals in alloys can enhance strength and durability, while semiconductors in the periodic table are crucial for developing electronic devices.
Environmental Studies:
The periodic table aids in environmental studies by providing insights into the behavior of elements in the environment. It helps scientists monitor pollution levels, study the impact of elements on ecosystems, and develop methods for environmental remediation and waste management.
Education and Learning:
The colorful and visually appealing layout of the periodic table makes it an effective educational tool. It helps students grasp the relationships between elements, learn about their properties, and understand the underlying patterns and trends. The periodic table is a foundation for teaching chemistry and serves as a reference throughout academic and professional careers.
Industrial Applications:
The periodic table is essential for various industrial sectors. It guides the selection of elements and compounds for specific applications, such as in pharmaceuticals, agriculture, energy production, and manufacturing. For instance, the knowledge of transition metals’ properties helps in designing catalysts for chemical reactions and developing efficient energy storage systems.
Research and Discovery of New Elements:
The periodic table is not static; it continues to expand as new elements are discovered. Scientists use the periodic table as a framework to guide their search for and identification of new elements. By studying the periodic trends and properties of known elements, researchers can make predictions and conduct experiments to unveil elements that fill the gaps in the table.
Conclusion
The periodic table is a remarkable achievement in scientific organization and a captivating representation of the elements that compose our universe. With its colorful layout and wealth of information, it serves as a valuable tool for scientists, educators, and learners alike. By understanding the periodic table, we gain insights into the building blocks of matter, uncover the secrets of chemical reactions, and drive innovation in various fields of science and technology.
Continued
The periodic table is not only a symbol of scientific progress but also a testament to the unity and diversity of the elements. It showcases the interconnectedness of the elements and their shared fundamental properties, while also highlighting their unique characteristics and behaviors.
As scientists continue to explore and expand our understanding of the elements, the periodic table evolves and adapts. New discoveries and advancements in scientific research contribute to the refinement of the table, ensuring its accuracy and relevance in the ever-changing landscape of chemistry.
Whether you’re a student, a scientist, or simply curious about the world around us, the periodic table remains an invaluable resource. Its colorful layout and organized structure invite us to explore the vast realm of elements and their intriguing properties.
So, next time you encounter the periodic table, take a moment to appreciate its beauty and significance. Behind its captivating colors and arrangement lies a wealth of knowledge that continues to inspire scientific breakthroughs and shape our understanding of the natural world.
In conclusion, the periodic table serves as a guiding light in the realm of chemistry, illuminating our path towards discovery, innovation, and a deeper appreciation of the elements that make up our universe.
Continued
As our understanding of chemistry and the elements deepens, the periodic table continues to play a crucial role in shaping scientific advancements and discoveries. Here are a few areas where the periodic table continues to make significant contributions:
Element Synthesis and Exploration:
Scientists are constantly pushing the boundaries of the periodic table by synthesizing new elements through particle accelerators and nuclear reactions. These synthetic elements often have short lifespans and are highly unstable, but their creation expands our knowledge of nuclear physics and allows us to explore the limits of the periodic table.
Isotopes and Radioactive Decay:
The periodic table provides valuable information about the isotopes of various elements. Isotopes are atoms of the same element with different numbers of neutrons, resulting in variations in their atomic mass. The study of isotopes and their radioactive decay plays a crucial role in fields such as radiology, archaeology, and environmental science.
Periodic Trends and Predictive Power:
By examining the periodic table, scientists can identify and understand periodic trends, which are patterns in element properties that vary across periods and groups. These trends help predict and explain the behavior of elements, such as atomic size, ionization energy, electronegativity, and reactivity. They serve as a foundation for making informed predictions about the properties of new and undiscovered elements.
Applications in Medicine:
The periodic table has numerous applications in medicine and healthcare. Many elements and their compounds are used in diagnostic imaging techniques, such as X-rays (using the element tungsten) and MRI (using gadolinium). Elements like iodine and technetium are utilized in medical isotopes for diagnostic scans and cancer treatment. Additionally, elements like platinum and gold have pharmaceutical uses in chemotherapy drugs.
Environmental Impact and Sustainability:
The periodic table helps scientists understand the environmental impact of elements and their compounds. It aids in studying the behavior of pollutants, identifying environmentally friendly alternatives, and developing sustainable practices. By analyzing the properties and reactivity of elements, researchers can work towards minimizing pollution, managing waste, and developing cleaner technologies.
Global Collaboration and Standardization:
The periodic table serves as a universal language for scientists and researchers worldwide. It provides a standardized framework for communication, enabling seamless collaboration and sharing of knowledge. The organization and structure of the periodic table ensure consistency and clarity across scientific disciplines, promoting efficient data exchange and understanding.
Conclusion
The periodic table continues to be a fundamental pillar of modern science. Its colorful layout, structured organization, and comprehensive information have revolutionized the way we study and understand the elements. The periodic table’s influence extends beyond the realm of chemistry, impacting fields such as physics, biology, medicine, materials science, and environmental research.
As we delve deeper into the complexities of the elements, the periodic table remains an essential tool that guides us in unlocking the mysteries of the universe. It sparks curiosity, facilitates discoveries, and provides a framework for ongoing exploration and innovation. The periodic table stands as a testament to human ingenuity and our relentless pursuit of knowledge.
So, let us continue to marvel at the beauty and significance of the periodic table, celebrating its contributions to science and embracing its ever-evolving nature.





